Medical Mark® speical laser design for medical devices and pharmaceutical manufacturers, custom engineering services available for ensure FDA compliance.
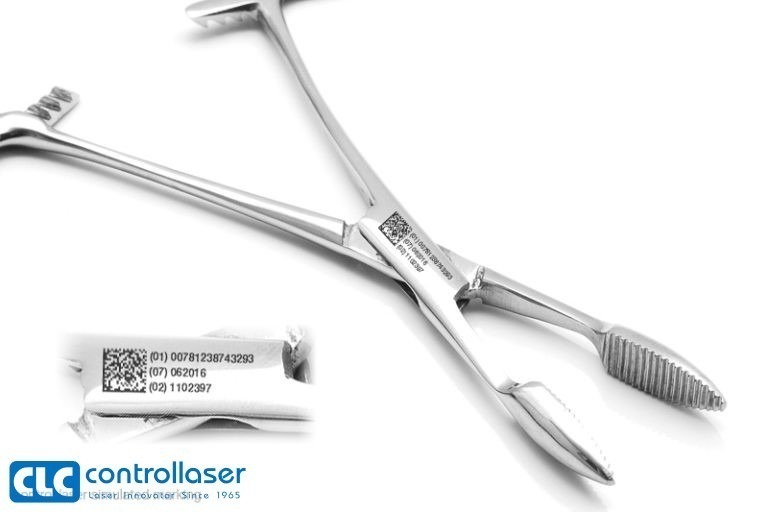
Medical Mark® is the solution for UDI laser coding and ERP data integration, compliant with code regulations by GS1, HIBCC, ICCBBA, sterile guaranteed quality.
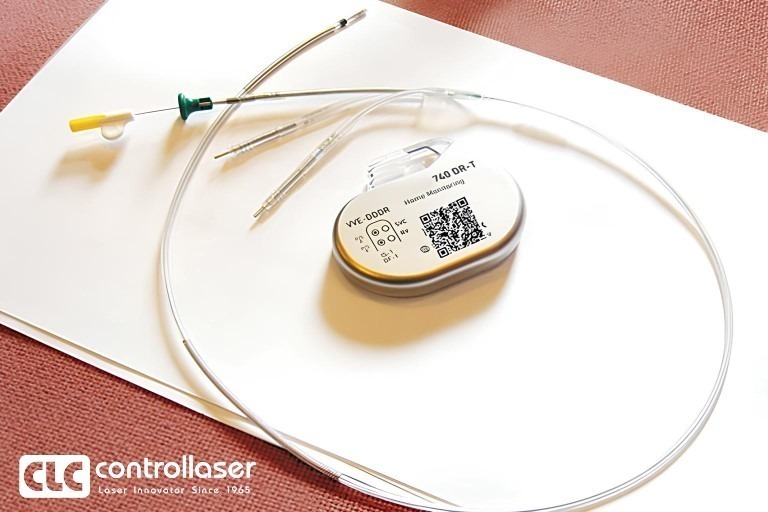
CLC’s laser marking and ablating technology for medical devices rooted from the laser marking system customized for DePuy in 1986. Now it has been developed as a complete UDI compliance laser coding application solution.
Trademark registered in 2014

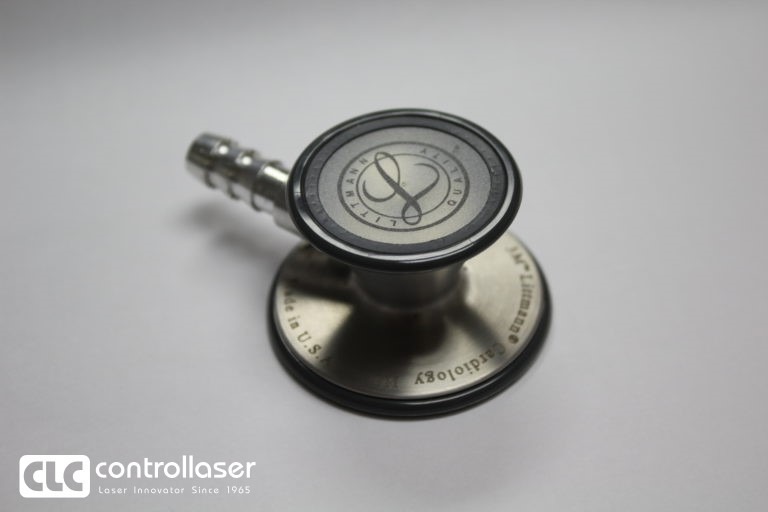
Application can fully or partially replace the traditional peening, roll stamping, grooving, embossing, stippling, scribing, sand blasting, and chemical etching, inkjet, printing process.
It provides the solution of regulation compliant high-quality marking and deep engraving process, helping the equipment owner leverage capital investment into good revenue generation.
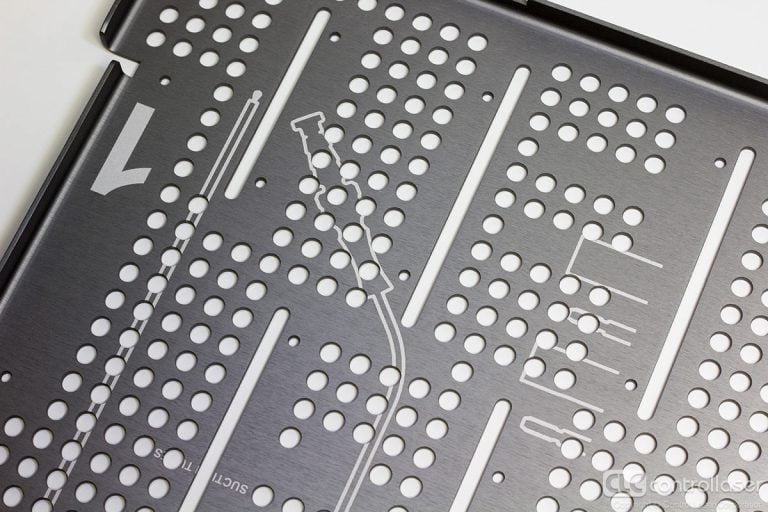






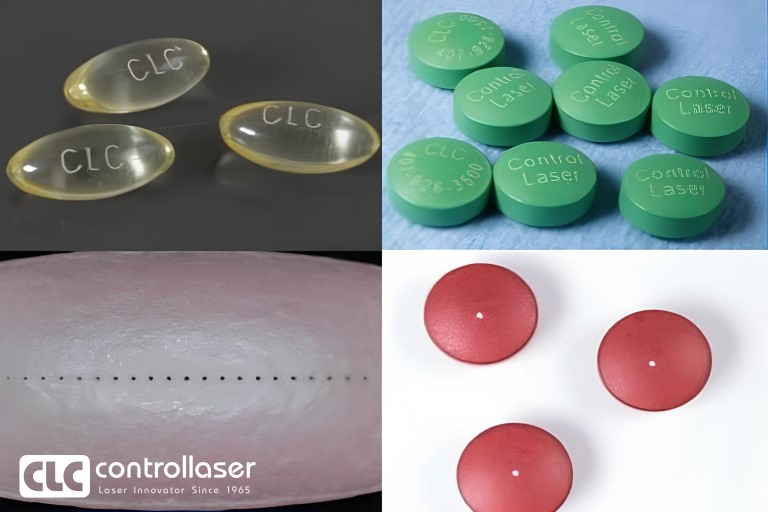
With short-wavelength or ultrafast laser sources, Medical Mark laser system can process tablet, gel capsule, Granules, and coating drilling for sustained release.
New design since 2019, we offer the pause and batch drilling technology, improve the productivity and yield more than 50%.
With this laser micromachining technology, users can resolve many challenges in processing new and special materials. The extreme ability of laser technology can reach helps the equipment owner significantly leverage capital investment into good revenue and margin generation.
